Geneweb
General References
Birstein VJ. Hanner R. DeSalle R. 1997. Phylogeny of the Acipenseriformes: cytogenetic and molecular approaches. Env. Biol. Fish. 48: 127-155 Abstract
Birstein VJ. Vasilev VP. 1987. Nucleolar organizer regions, their function and polyploidy in fishes. Zhurnal Obshchei Biologii 48 (6): 729-747 Abstract
Fontana F. 2002. A cytogenetic approach to the study of taxonomy and evolution in sturgeons. J. Appl. Ichthyol. 18: 226-233. (Table I, Table II) Abstract
Fontana F. Lanfredi M. Congiu L. Leis M. Chicca M. Rossi R. 2003. Chromosomal mapping of 18S-28S and 5S rRNA genes by two-colour fluorescent in situ hybridization in six sturgeon species. Genome 46: 473-477 Abstract
Fontana F. Rossi R. Lanfredi M. Arlati G. Bronzi P. 1999. Chromosome banding in sturgeons. J. Appl. Ichthyol. 15: 9-11 Abstract
Vasil’ev VP. 1999. Polyploidization by reticular speciation in Acipenseriform evolution: a working hypothesis. J. Appl. Ichthyol. 15: 29–31. Abstract
Fontana F. Tagliavini J. Congiu L. 2001. Sturgeon genetics and cytogenetics: recent advancements and perspectives. Genetica 111: 359-373 Abstract
Fontana F. Zane L. Pepe A. 2007. Congiu L. Polyploidy in Acipenseriformes: cytogenetic and molecular approaches. In Fish Cytogenetics. Eds: E. Pisano, C. Ozouf-Costaz, F. Foresti & B.G. Kapoor. Science Publisher, Inc. New Hampshire, USA. pp. 385-403
Havelka M., Kašpar V., Hulák M., Flajšhans M. 2011. Sturgeon genetics and cytogenetics: a review related to ploidy levels and interspecific hybridization. Folia Zool. 60: 93-103 Abstract
Lanfredi M. Congiu L. Garrido-Ramos MA. De La Herrán R. Leis M. Chicca M. Rossi R. Tagliavini J. Ruiz Rejón C. Ruiz Rejón M. Fontana F. 2001. Chromosomal location and evolution of a satellite DNA family in seven sturgeon species. Chrom. Res. 9: 47-52 Abstract
Robinson M R. Ferguson M M. 2004. Genetics of North American Acipenseriformes. In: Sturgeons and Paddlefish of North America. Edited by. LeBreton Greg TO, Beamish F William H, McKinley R. Scott. Kluwer Academic Publishers, Dordrecht, Boston. Chapter 10, pp. 217-230 Abstract
Vasiliev VP. 1985. Evolutionary karyology of fishes. Nauka Press, Moscow. 126-139. (in Russian).
Vasil’ev VP. 2009. Mechanisms of polyploid evolution in fish: Polyploidy in Sturgeons. In: Biology, Conservation and Sustainable Development of Sturgeons. Eds: R. Carmona, A. Domezain, M. García-Gallego, J. A. Hernando, F. Rodríguez, M. Ruiz-Rejón. Springer Science + Business Media BV: pp. 97-117 Abstract
Nikolsky G. 1976. The interrelation between variability of characters, effectiveness of energy utilisation, and karyotype structure in fishes. Evolution 30: 180-185 Abstract
Vasiliev VP. Sokolov L. 1980. The method for studying of chondrostean karyotypes. Tsitologia (Leningrad) 22, 1106-1109 (in russian) Abstract
Vasil’eva ED. Vasil’ev VP. Shedko SV. Novomodny GV. 2009. The Revision of the validity of genus Huso (Acipenseridae) based on recent morphological and genetic data with particular reference to the kaluga H. dauricus. Journal of Ichthyology, 49(10): 861–867. Abstract
Zeng L. Xiao Y. Li X. Zhou Y. Fan Y. 2009. Establishment and characterization of a cell line derived from fin of paddlefish, Polyodon spathula Walbaum. 6th International Symposium on Sturgeon. October 25-31, Wuhan, Hubei Province, China. Posters A58. Abstract
Genome Size
Mirsky AE. Ris H. 1951. The desoxyribonucleic acid content of animal cells and its evolutionary significance. J. Gen. Physiol. 34: 451-462.
(A. sturio Cv 3.2)
Vialli M. 1957. Volume et contenu en ADN par noyau. Exp.Cell Res. Suppl. 4: 284-293.
(A. naccarii Cv 4.24; A. sturio Cv 3.95)
Kafiani KA. RI Tatarskaia. Kanopkaite SM. 1958. Phosphorus metabolism in the embryonic development of sturgeon. Biochemistry 23: 389-399.
(A. stellatus Cv 4.70)
Ohno S. Muramoto J. Stenius C. Christian L. Kitterell WA. 1969. Microchromosomes in holocephalian, chondrostean and holostean fishes. Chromosoma 26: 35-40 Abstract
(S. platorynchus Cv 3.60)
Fontana F. 1976. Nuclear DNA content and cytometric of erythrocytes of Huso huso L., Acipenser sturio L. and Acipenser naccarii Bonaparte. Caryologia 29: 127-138 Abstract
(H. huso Cv 3.60; A. naccarii Cv 6.30; A. sturio Cv 3.58)
Hinegardner R. 1976. The cellular DNA content of sharks, rays and some other fishes. Comp. Biochem. Physiol., B. 55: 367-370.
(A. transmontanus Cv 10.60)
Tiersch TR. Chandler RW. Wachtel SS. Elias S. 1989. Reference standards for flow cytometry and application in comparative studies of nuclear DNA content. Cytometry 10: 706-710 Abstract.
(P. spathula Cv 3.90)
Blacklidge KH. Bidwell CA. 1993. Three ploidy levels indicated by genome quantification in Acipenseriformes of North America. J. Hered. 84: 427-430 Abstract
(A. brevirostrum C.v. 13.07; A. fulvescens C.v. 8.90; A. medirostris Cv 8.82; A. oxyrhynchus Cv 4.55; A. transmontanus Cv 9.46; S. platorynchus Cv 4.73; P. spathula Cv 4.89)
Birstein VJ. Poletaev AI. Goncharov BF. 1993. The DNA content in Eurasian sturgeon species determined by flow cytometry. Cytometry, 14(4): 337-383 Abstract
(H. dauricus Cv 3.77; H.huso Cv 3.43; A. baeri Cv 8.30; A. gueldenstaedtii Cv 7.87; A. medirostris Cv 14.33; A. nudiventris Cv 3.96; A. ruthenus Cv 3.74; A. stellatus Cv 3.74; P.kaufmanni Cv 3.47; P. spathula Cv 3.17)
Song S. Liu H. Sun D. Fan Z. 1997. The karyotype and cellular DNA contents of Amur sturgeon (Acipenser schrencki). Hereditas (Beijing). 19: 5-8 (in Chinese). Abstract
(A. schrenckii Cv 11.73)
Zhang SM. Yang Y. Deng H. Wei QW. Wu QJ. 1999. Genome size and ploidy characters of several species of sturgeons and paddlefishes with comments on cellular evolution of Acipenseriformes. Acta Zoologica Sinica, 45(2): 200-206 Abstract
(P. gladius Cv 4.11; A. dabryanus Cv 8.26; A. sinensis Cv 9.07; A. schrenckii Cv 6.07; P. spathula Cv 3.96)
Hardie DC. Hebert PDN. 2003. The nucleotypic effects of cellular DNA content in cartilaginous and ray-finned fishes. Genome 46: 683-706 Abstract
(A. brevirostrum Cv 13.78; A. oxyrhynchus Cv 4.38)
Hardie DC. Hebert PDN. 2004. Genome-size evolution in fishes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1636-1646. Abstract
(A. brevirostrum Cv 13.78; A. oxyrhynchus Cv 4.38)
Yin HB. Sun ZW. Sun DJ. 2004. Comparative study on DNA contents of five cultured fishes of Acipenseridae and Huso. Journal of Shanghai Fisheries University 13, 111–114. Abstract
(A. gueldenstaedtii Cv 12.24; A.baeri Cv 11.60; A. schrenckii Cv 11-59; A.ruthenus Cv 6.06; H. dauricus Cv 4.77)
(A. baeri Cv 7.8-8.0; A.fulvescens Cv 7.9-8.0; A. gueldenstaedtii Cv 4.2/8; A. medirostris mikadoi Cv 8.0-9.1; A. ruthenus Cv 3.8-3-9; A. schrenckii Cv. 7.9-8.2; A. stellatus Cv 3.5-4.0; A. transmontanus Cv 8.5-9.0; H. dauricus Cv 8.3-8.4; P. spathula Cv 3.5) Abstract
Zhou H. Fujimoto T. Adachi S. Yamaha E. Arai K. 2011. Genome size variation estimated by flow cytometry in Acipenser mikadoi, Huso dauricus in relation to other species of Acipenseriformes. J. Appl. Ichthyol. 27: 484-491-
(A. mikadoi Cv 8.2; H. dauricus Cv 8,3) Abstract
Zhou H. Fujimoto T. Adachi S. Abe, S. Yamaha E. Arai K. 2013. Molecular cytogenetic study on the ploidy status in Acipenser mikadoi. Abstract
Zhou H. Fujimoto T. Adachi S. Abe, S. Yamaha E. Arai K. 2013. Molecular cytogenetic study on the ploidy status in Acipenser mikadoi. J. Appl. Ichthyol. 29: 51-55 (A. mikadoi Cv 8.82) Abstract
Acipenser baerii
| 2n ~ 209 Burtzev JA. Nikoljukin NJ. Serebryakova EV. Karyotype presented in 1973 at the First European Ichthyological Congress, held in Sarajevo by the the Yugoslav Society of Ichthyology (personal communication to Francesco Fontana) |
 |
| 2n=249±5; 120 mc 308±4 FN Vasil’ yev VP. Sokolov LI. Serebryakova EV. 1980. Karyotype of the Siberian sturgeon Acipenser baeri Brandt from the Lena River and some questions of the acipenserid karyotypic evolution. Vopr Ikhtiol 23: 814-822 Abstract |
 |
| Vasiliev VP. 1985. Evolutionary karyology of fishes. Nauka Press, Moscow. 126-139. (in Russian). | |
| 2n=246±8; 98 m+sm, 150 a+mc, 346 FN Fontana F. 1994. Chromosomal nucleolar organizer regions in four sturgeon species as markers of karyotype evolution in Acipenseriformes (Pisces). Genome 37: 888-892 Abstract |
 |
|
2n=246±10 |
|
|
Fontana F. Lanfredi M. Chicca M. Aiello V. Rossi R. 1998. Localization of the repetitive telomeric sequence (TTAGGG)n in four sturgeon species. Chrom. Res. 6: 303-306 Abstract |
|
|
2n=229-240 |
|
| Havelka M. Hulák M. Ráb P. Rábová M. Lieckfeldt D. Ludwig A. Rodina M. Gela D. Pšenička M. Bytyutskyy D. Flajšhans M. 2014. Fertility of a spontaneous hexaploid male Siberian sturgeon, Acipenser baerii. BMC Genetics, 15 Abstract |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser brevirostrum
| 372 (254-372); 178 m+sm, 194 a+mc, 550 FN Kim DS. Nam YK. Noh JK. Park CH. Chapman FA. 2005. Karyotype of North American shortnose sturgeon Acipenser brevirostrum with the highest chromosome number in the Acipenseriformes. Ichthyol. Res. 52: 94–97 Abstract |
 |
| Flynn SR, Matsuoka M, Reith M, Martin-Robichaud DJ, Benfey TJ 2006. Gynogenesis and sex determination in shortnose sturgeon, Acipenser brevirostrum Lesuere. Aquaculture 253: 721-727 Abstract | |
| 372±6; 178 m+sm, 196 a+mc, 468 FN Fontana F, Congiu L, Mudrak VA, Quattro JM, Smith TIJ, Ware K, Doroshov SI. 2008- Evidence of hexaploid karyotype in shortnose sturgeon- Genome 51(2): 113-119. DOI 10.1139/G07-112 Abstract (Table 1, Table 2) |
 |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser fulvescens
| 2n=262±6; 134 m+sm, 70 t+a, ~60 mc, ~468 FN Fontana F. Bruch RM. Binkowski FP. Lanfredi M. Chicca M. Beltrami N. Congiu L. 2004. Karyotype characterization of the lake sturgeon, Acipenser fulvescens (Rafinesque, 1817) by chromosome banding and fluorescent in situ hybridization. Genome 47: 742-746. Abstrac |
m = metacentric; sm = submetacentric; t = telocentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser gueldenstaedtii
| 2n ~ 187 Burtzev JA. Nikoljukin NJ. Serebryakova EV. Karyotype presented in 1973 at the First European Ichthyological Congress, held in Sarajevo by the the Yugoslav Society of Ichthyology (personal communication to Francesco Fontana) |
|
| 2n=250±8; 92±4 m+sm, 150 st+a+mc, 342±12 FN Vasiliev VP. 1985. Evolutionary karyology of fishes. Nauka Press, Moscow. 126-139. (in Russian). |
|
|
2n=250±8, 92±4 m+sm, (250±8 + 92)±4 FN |
 |
| 2n=249.9±2.2; 97.6±0.4 m, 152.2±2.6 a, 346.1±2.3 FN Arefjev VA. Nikolaev AI. 1991. Cytological analysis of the reciprocal hybrids between low- and high-chromosome acipenserids, the great sturgeon, Huso huso (L.), and the Russian sturgeon, Acipenser gueldenstaedti Brandt. Cytologia 56: 495-502 Abstract |
|
|
2n=250±; 98 m+sm, 152 a+mc, 348 |
|
|
n=256±8 |
|
| 2n=258±4; 106 m+sm, 152 a, 364±8 FN Fontana F. Lanfredi M. Rossi R. Bronzi P. Arlati G. 1996 Karyotypic characterization of Acipenser gueldenstaedti with C-, AgNO3 and fluorescence banding techniques. Ital. J. Zool. 63: 113-118 Abstract |
|
| Fontana F. Lanfredi M. Chicca M. Aiello V. Rossi R. 1998. Localization of the repetitive telomeric sequence (TTAGGG)n in four sturgeon species. Chrom. Res. 6: 303-306 Abstract | |
|
2n=236±2; 96 m+sm, 140 st+a+mc, 332 FN |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser medirostris
| 2n=249±8 Van Eenennaam AL. Murray JD. Medrano JF. 1999. Karyotype of the American green sturgeon. Transactions of the American Fisheries Society 128: 175-177 Abstract |
 |
Acipenser mikadoi
|
2n=264
Vasil’ev VP. Vasil’eva ED. Shedko SV. Novomodny GV. 2008. Karyotypes of the kaluga Huso dauricus and Sakhalin sturgeon Acipenser mikadoi (Acipenseridae, Pisces). In: Bioraznoobrazie I dinamika genofondov. Materialy otchetnoi konferentsii. Moscow: RAN. P. 19-21
|
 |
|
2n=247±33
Vishnyakova KS. Mugue NS. Zelenina DA. Mikodina EV. Kovaleva OA. Madan GV. Yegorov YE. 2008. Cell culture and karyotype of Sakhalin sturgeon Acipenser mikadoi. Biologicheskie membrany. 25:420-433 Abstract
|
 |
|
2n=262±4; 80 m+sm, 342±4 FN
Vasil’ev VP. Vasil’eva ED. Shedko SV. Novomodny GV. 2009. Ploidy levels in the kaluga Huso dauricus and sakhalin sturgeon Acipenser mikadoi (Acipenseridae, Pisces). Doklady Biological Sciences, 426: 228-231
|
|
|
2n=262±4; 80 m+sm, 342±4 FN
Vasil’ev VP. Vasil’eva ED. Shedko SV. Novomodny GV. 2010. How many times has polyploidization
occurred during acipenserid evolution? New data on the karyotypes of sturgeons (Acipenseridae, Actinopterygii) from the Russian far east. Journal of Ichthyology, 50: 950-959. Abstract
|
|
|
Vasil’eva ED. Vasil’ev VP. Shedko SV. Novomodny GV. 2009. The validation of specific status of the Sakhalin sturgeon Acipenser mikadoi (Acipenseridae) in the light of recent genetic and morphological data. Journal of Ichthyology, 49(10): 868–873. Abstract
|
|
|
2n=248 |
|
| 2n=230-272; 80 m+sm, 188 a+mc, Zhou H. Fujimoto T. Adachi S. Abe, S. Yamaha E. Arai K. 2013. Molecular cytogenetic study on the ploidy status in Acipenser mikadoi. J. Appl. Ichthyol. 29: 51-55 Abstract |
Acipenser naccarii
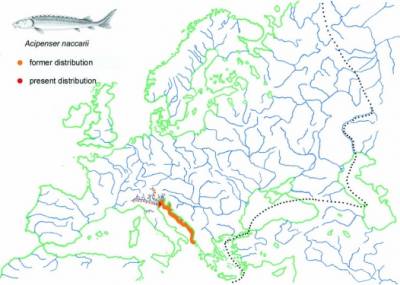
2n=239±7; 150 m+sm, 88 a+mc, 388 FN
Fontana F. Colombo G. 1974. The chromosomes of Italian sturgeons. Experientia 30: 739-742 Abstract
2n=246±8
Fontana F. 1994. Chromosomal nucleolar organizer regions in four sturgeon species as markers of karyotype evolution in Acipenseriformes (Pisces). Genome 37: 888-892 Abstract
2n=246±8
Fontana F. Lanfredi M. Rossi R. Bronzi P. Arlati G. 1995. Established cell lines from three sturgeon species. Sturg. Quart. 3(4): 6-7
| 2n=241±3; 88±2 m, 153 a+mc, 329±4 FN Arlati G. Belysheva LA. Kaidanova TI. 1995. Kariological analysis of Acipenser naccarii (Bonaparte). Proc. Intern. Sturg. Symp., VNIRO Publ. 119-123 Abstract |
 |
| Fontana F. Lanfredi M. Chicca M. Aiello V. Rossi R. 1998. Localization of the repetitive telomeric sequence (TTAGGG)n in four sturgeon species. Chrom. Res. 6: 303-306 Abstract | |
| 2n=248±4; 174 m+sm, 2 st, 72 a+mc, 424 FN Fontana F. Lanfredi M. Chicca M. Congiu L. Tagliavini J. Rossi R. 1999. Fluorescent in situ hybridization with rDNA probes on chromosomes of Acipenser ruthenus and Acipenser naccarii (Osteichthyes Acipenseriformes). Genome 42: 1008-1012 Abstract |
 |
| Congiu L., Pujolar JM. Forlani A. Cenadelli S. Dupanloup I. Barbisan F. Galli A. Fontana F. 2011. Managing Polyploidy in Ex Situ Conservation Genetics: The Case of the Critically Endangered Adriatic Sturgeon (Acipenser naccarii). PLoS ONE 6(3): e18249. doi:10.1371/journal.pone.0018249 Abstract |
m = metacentric; sm = submetacentric; st = subtelocentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser nudiventris
| 2n=118±3; 54±4 m, 4 a, 60±3 mc, 172±4 FN Arefjev VA. 1983. Polykaryogrammic analysis of ship, Acipenser nudiventris Lovetsky (Acipenseridae, Chondrostei). Vopr. Ikhtiol. 23: 209-218 Abstract |
|
| 2n=118±2; 54 m+sm, 64 a+mc, 172 FN Sokolov LI. Vasiliev V. 1989 Acipenser nudiventris Lovetsky, 1928 In: The freshwater fishes of Europe. Vol 1/II General introduction to fishes Acipenseriformes, Holcik J. Ed., Wiesbaden: 206-226 |
|
| 2n=116±4; 60±4 m+sm+st, 56mc, 120±4 FN Nowruzfashkhami MR. Safaiian S. Bahmani M. Chubian F. 2006. Karyotype analysis in ship sturgeon Acipenser nudiventris in the south Caspian Sea using leukocyte culture . J. Appl. Ichthyol. 22 (Suppl. 1): 97-98 Abstract |
m = metacentric; sm = submetacentric; st = subtelocentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser oxyrinchus
| 2n=99-112 Li MF. Marrayatt V. Annand C. Odense P. 1985. Fish cell culture: two newly developed cell lines from Atlantic sturgeon (Acipenser oxyrhynchus) and guppy (Poecilia reticulata). Can. J. Zool. 63: 2867-2874 Abstract |
|
|
2n=121±3; 78 m+sm, 44a+mc, 200 FN |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser persicus
| 2n>200 Nowruzfashkhami M. R. 1996. On the Karyotypes of Acipenser persicus, A. stellatus and Huso huso from the Iranian Waters of the Caspian Sea. Sturg. Quat. 4 (3): 7. |
|
|
258±4; 134 m+sm, 128 a+mc, 386 NF |
|
| Nowruzfashkhami MR. Kazemi B, Jahani M, Pourkazemi M, Wazeeri-Nasab H, Azizzadeh L. 2009. Chromosomal location of satellite DNA family in Acipenser persicus. 6th International Symposium on Sturgeon. October 25-31, Wuhan, Hubei Province, China. Book of Abstracts, Oral Presentations 77-78. Abstract |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser ruthenus
| 2n=60±2; 40 m+sm, 20 a, 100 FN Only macrochromosomes were counted Serebryakova EV. 1972. Some data on the chromosome complexes in Acipenseridae.. In: Genetics, Selection, and Hybridization of Fish. (Ed. B.I. Cherfas). Translated from Russian by Israel Program for Scientific Translations. Keter Press Binding: Wiener Bindery Ltd. Jerusalem. pp. 98-106 |
|
|
2n=116±4; 66 m+sm, 40 a+mc, 172 FN |
|
|
2n=109-112; 159-165 NF |
|
| 2n=118±2; 82 m+sm, 36 a+mc, 200 FN Vasiliev VP. 1985. Evolutionary karyology of fishes. Nauka Press, Moscow. 126-139. (in Russian). |
|
| 2n=118±4; 58 m+sm, 4 a, 56±4 mc, 176±4 FN Rab P. 1986. A note on the karyotype on the sterlet, Acipenser ruthenus (Pishes, Acipenseridae). Folia Zool. 35(1): 73-78 Abstract |
|
| 2n=118±2; 82±4 m+sm, 118±2 + 82±4 FN Birstein VJ. Vasiliev VP. 1987. Tetraploid-octoploid relationships and karyological evolution in the order Acipenseriformes (Pishes): karyotypes, nucleoli, and nucleolus-organizer regions in four acipenserid species. Genetica 73: 3-12 Abstract |
 |
| 2n=117.3±0.6; 57.2±0.3 m, 60.1±0.7 a, 174.6±0.7 FN Arefjev VA. 1989. Karyotype variability in successive generations after hybridization between the great sturgeon, Huso huso (L.), and the sterlet, Acipenser ruthenus. L. J. Fish Biol. 35: 819-828 Abstract |
|
|
2n=118±4 |
|
|
2n=118±9 |
|
| 2n=118±4; 58 m+sm, 4a, 52-60 mc Ráb P. Arefjev VA. Rábova M. 1996. C-banded karyotype of the sterlet, Acipenser ruthenus, from the Danube River. Sturg. Quart., 4(4): 10-12 Abstract |
 |
| Fontana F. Lanfredi M. Chicca M. Aiello V. Rossi R. 1998. Localization of the repetitive telomeric sequence(TTAGGG)n in four sturgeon species. Chrom. Res. 6: 303-306 Abstract | |
| 2n=118±2; 58 m+sm, 4a, 56±2mc Suciu R. Ene C. 1998. A note on the karyotype of the sterlet Acipenser ruthenus Linnaeus, 1758 (Pisces, Acipenseridae) from the romanian stretch of Danube River. Extended abstracts of contributions presented at the International Symposium Aquarom 98/Galati: 318-321 Abstract |
 |
| 2n=118±4 Fontana F. Lanfredi M. Chicca M. Congiu L. Tagliavini J. Rossi R. 1999. Fluorescent in situ hybridization with rDNA probes on chromosomes of Acipenser ruthenus and Acipenser naccarii (Osteichthyes Acipenseriformes). Genome 42: 1008-1012 Abstract |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes FN = fondamental number
Acipenser sinensis
| 2n=264±; 78 m, 20 sm, 166 a+mc, 362 FN Yu X. Zhou T. Li K. Li Y. and Zhou M. 1987. On the karyosystematics of cyprinid fishes and a summary of fish chromosome studies in China. Genetica 72: 225-236 Abstract |
 |
| 2n=156-276 Ye XH, Liu HQ, Yu XM, Zhang YB, Chang JB.1999. Preliminary research on tissue culture of Chinese sturgeon. Acta Hydrobiology Sinica 23, 566-571. (In Chinese) Abstract |
|
|
2n=264 (mode) |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser schrenckii
| 2n=240 Vasiliev VP. Sokolov LI. Serebryakova EV. 1980. Karyotype of the Siberian sturgeon Acipenser baeri Brandt from the Lena River and some questions of the acipenserid karyotypic evolution. Vopr. Ikhtiol. 23: 814-822 Abstract |
|
| 2n=238±8; 84 m+sm, 28 st, 120 a+mc, 328 FN Song, S. Liu H. Sun D. Fan Z. 1997. The karyotype and cellular DNA contents of Amur sturgeon (Acipenser schrencki). Hereditas (Beijing). 19: 5-8 (in Chinese). Abstract |
 |
|
2n=266±4; 92 m+sm, 358±4 FN |
m = metacentric; sm = submetacentric; st = subtelocentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser sturio
|
2n=116±4; 70 m+sm, 42a+mc, 182 FN |
|
| 2n=121±3; 78 m+sm, 43a+mc, 198 FN Tagliavini J. Williot P. Congiu L. Chicca M. Lanfredi M. Rossi R. Fontana F. 1999. Molecular cytogenetic analysis of the karyotype of the European Atlantic sturgeon, Acipenser sturio. Heredity 83: 520-525 Abstract |
|
|
Fontana F. 2011. Cytogenetics as a tool for an exploration of A. sturio status within sturgeons. In Biology and Conservation of the European Sturgeon Acipenser sturio L. 1758. Eds P. Williot, E. Rochard, N. Desse-Berset, F. Kirschbaum & J. Gessner. Springer-Verlag Berlin Heidelberg. pp. 13-21 Abstract |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Acipenser stellatus
| 2n=109-112; 159-165 NF Burtzev JA. Nikoljukin NJ. Serebryakova EV. 1976. Karyology of the Acipenseridae family in relation to the hybridization and taxonomy problems. Acta Biol. Jugosl. Ser. Ichthyologia 8: 27-34 Abstract |
|
| 2n=118±2; 70 m+sm, 48 a+mc, 188 FN Vasiliev VP. 1985. Evolutionary karyology of fishes. Nauka Press, Moscow. 126-139. (in Russian). |
 |
| 2n=118±2; 70±4 m+sm, (118±2)+(70±4) FN Birstein VJ. Vasiliev VP. 1987. Tetraploid-octoploid relationships and karyological evolution in the order Acipenseriformes (Pishes): karyotypes, nucleoli, and nucleolus-organizer regions in four acipenserid species. Genetica 73: 3-12 Abstract |
|
|
2n=118±1, 356 FN |
|
| 2n=118±2; 186±2 FN Suciu R. Ene C. 1996. Karyological study of the stellate sturgeon, Acipenser stellatus, from the Danube River. Sturg. Quart. 4(3): 14-15 |
|
|
2n=114 |
|
|
2n=146±6; 72 m+sm, 74 a+mc, 218 FN |
 |
m = metacentric; sm = submetacentric chromosomes; FN = fondamental number
Acipenser transmontanus
| 2n=237-243 Hedrick RP. McDowell TS. Rosemarck R. Aronstein D. Lannan C.N. 1991. Two cell lines from white sturgeon. Trans. Am. Fish Soc. 120: 528-534 Abstract |
|
| 2n=248±8; 104 m+sm, 144 a+mc, 352 FN Fontana F. 1994. Chromosomal nucleolar organizer regions in four sturgeon species as markers of karyotype evolution in Acipenseriformes (Pisces). Genome 37: 888-892 Abstract |
 |
| 2n=226-288 Sola L. Cordisco C. Bressanello S. Cataudella S. 1994. Cytogenetic characterization of the North American white sturgeon Acipenser transmontanus (Pisces, Acipenseridae). In: Proc. VIII Congr. SEI: 64-65 Abstract |
|
|
2n=246±10 |
|
| Vaneenennaam AL. Murray JD. Medrano JF. 1998. Synaptonemal complex analysis in spermatocytes of white sturgeon, Acipenser transmontanus Richardson (Pisces, Acipenseridae), a fish with a very high chromosome number. Genome 41: 51-61 Abstract |  |
| 2n=271±2.5; 132 m+sm, 44 a, 98 mc, 306 FN Vaneenennaam AL. Murray JD. Medrano JF. 1998. Mitotic analysis of the North American white sturgeon, Acipenser transmontanus Richardson (Pisces, Acipenseridae), a fish with a very high chromosome number. Genome 41:266-271 Abstract |
 |
| 2n=256±6 Wang G. Lapatra S. Zeng L. Zhao Z. Lu Y. 2003. Establishment, growth, cryopreservation and species of origin identification of three cell lines from white sturgeon, Acipenser transmontanus. Meth. Cell. Sci. 25: 211–220 Abstract |
|
| Gille DA.Famula TR.May BP.Schreier AD. 2015. Evidence for a maternal origin of spontaneous autopolyploidy in cultured white sturgeon (Acipenser transmontanus). Aquaculture, 435: 467-474 Abstract |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Polyodon spathula
| 2n=120; 32 m, 8 sm, 8 a, 72 mc, 160 FN Dingerkus G. Howell WM. 1976. Karyotypic analysis and evidence of tetraploidy in the North American paddlefish, Polyodon spathula. Science 194: 842 844 Abstract |
 |
|
2n=120; 48 m+sm, 72 mc |
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Scaphirhynchus platorynchus
| 2n=112; 50 m, 14 a, 48 mc, 162 FN Ohno S. Muramoto J. Stenius C. Christian L. Kitterell WA. 1969. Microchromosomes in holocephalian, chondrostean and holostean fishes. Chromosoma 26: 35-40 Abstract |
m = metacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Huso dauricus
| 2n=60 Only macrochromosomes were counted Burtzev JA. Nikoljukin NJ. Serebryakova EV. 1973 Kariology of family Acipenseridae in connection with questions of hybridization and taxonomy. First European Ichthyological Congress. Sarajevo. Yugoslavia |
|
| 2n=270 Vasil’ev VP. Vasil’eva ED. Shedko SV. Novomodny GV. 2008. Karyotypes of the kaluga Huso dauricus and Sakhalin sturgeon Acipenser mikadoi (Acipenseridae, Pisces). In: Bioraznoobrazie I dinamika genofondov. Materialy otchetnoi konferentsii. Moscow: RAN. P. 19-21 |
 |

2n=268±4; 100 m+sm, 368±4 FN Vasil’ev VP. Vasil’eva ED. Shedko SV. Novomodny GV. 2009. Ploidy levels in the kaluga Huso dauricus and sakhalin sturgeon Acipenser mikadoi (Acipenseridae, Pisces). Doklady Biological Sciences, 426: 228-231 |
 |
|
2n=268±4; 100 m+sm, 368±4 FN |
Huso huso
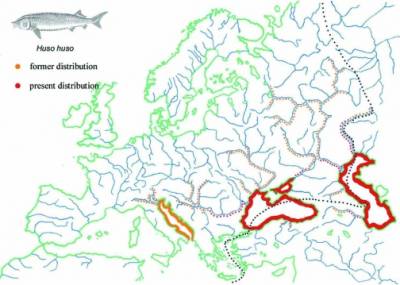
| 2n=60±2; 38 m+sm, 22 a, 98 FN Only macrochromosomes were counted Serebryakova EV. 1972. Some data on the chromosome complexes in Acipenseridae.. In: Genetics, Selection, and Hybridization of Fish. (Ed. B.I. Cherfas). Translated from Russian by Israel Program for Scientific Translations. Keter Press Binding: Wiener Bindery Ltd. Jerusalem. pp. 98-106 |
|
| 2n=116±4; 68 m+sm, 46 a, 182 FN Fontana F. Colombo G. 1974. The chromosomes of Italian sturgeons. Experientia 30: 739-742 Abstract |
|
| 2n=109-112; 159-165 NF Burtzev JA. Nikoljukin NJ. Serebryakova EV. 1976. Karyology of the Acipenseridae family in relation to the hybridization and taxonomy problems. Acta Biol. Jugosl. Ser. Ichthyologia 8: 27-34 Abstract |
| 2n=118±3; 62±4 m, 6 a, 50±3 mc, 180±4 FN Serebryakova EV. Arefjev VA. Vasiliev VP. Sokolov L.I. 1983. The study of the karyotype of giant sturgeon, Huso huso (L.) (Acipenseridae , Chondrostei) with reference to their systematic position. In Genetics of Commercial Fishes and Aquaculture Objects. pp 63-69, Moscow Abstract |
 |
| Arefjev VA, Panov AD. 1984. Some problems of the chromosome distribution in the metaphase plates of great sturgeon, Huso huso (L) Genetika 20 (8): 1374-1379 1984 Abstract | |
| 2n=118±2; 60±2 m+sm, (118±2)+(60±2) FN Birstein VJ. Vasiliev VP. 1987. Tetraploid-octoploid relationships and karyological evolution in the order Acipenseriformes (Pishes): karyotypes, nucleoli, and nucleolus-organizer regions in four acipenserid species. Genetica 73: 3-12 Abstract |
 |
| 2n=118.6±0.5; 61.5±0.3 m, 57.9±0.5 a, (177.9±0.6) FN Arefjev VA. 1989. Karyotype variability in successive generations after hybridization between the great sturgeon, Huso huso (L.), and the sterlet, Acipenser ruthenus. L. J. Fish Biol. 35: 819-828 Abstract |
|
| 2n=117.6±0.4; 61.2±0.2 m, 57.6±0.6 a, (178.2±0.5) FN Arefjev VA. Nikolaev AI. 1991. Cytological analysis of the reciprocal hybrids between low- and high-chromosome acipenserids, the great sturgeon, Huso huso (L.), and the Russian sturgeon, Acipenser gueldenstaedti Brandt. Cytologia 56: 495-502 Abstract |
 |
| Arefjev VA. 1993. NOR-banding studies of Acipenser baeri karyotype. Abstract Bulletin Internatrional Symposium on sturgeons Moscow, September 6-11, 1993. VNIRO, Moscow-Kostroma-Moscow, Russia. pp 30-31 Abstract | |
|
2n=116±1, 356 FN |
|
| 2n=120±8 Fontana F. Rossi R. Lanfredi M. Arlati G. Bronzi P. 1997. Cytogenetic characterization of cell lines from three sturgeon species. Caryologia 50: 91-95 Abstract |
|
| 2n=118±2; 84 m+sm, 34 a+mc, 202 FN Fontana F. Tagliavini J. Congiu L. Lanfredi M. Chicca M. Laurenti C. Rossi R. 1998 - Karyotypic characterization of the great sturgeon, Huso huso, by multiple staining techniques and fluorescent in situ hybridization. Mar. Biol. 132: 495-501 Abstract |
|
| 2n=117 Nowruz Fashkhami MR. Khosroshahi M. 1999. Karyotype study on stellate and great sturgeon by leukocyte culture. J. Appl. Ichthyol. 15: 283. Abstract |
Hybrids
|
|
|
|
Nikoljukin N.I. 1966. Some questions of cytogenetics, hybridization and systematics of the Acipenseridae. Genetika (USSR), 5: 25-27 Abstract |
|
|
Arefjev, V.A. 1989. Increase of karyotypic variability iomn sturgeon hybrids. 6 p. In: Book of Abstracts, Premier Colloque International Sur L`esturgeon, Bordeaux, 3-6. October 1989. Published by Cemagref, 98 pp Abstract |
|
| Arefjev VA. 1999. Cytogenetics of interploid hybridization of sturgeons. J. Appl. Ichthyol. 15: 277 Abstract | |
|
Flajšhans M. Vajcová V. 2000. Odd ploidy levels in sturgeon suggest a backcross of interspecific hexaploid sturgeon hybrids to evolutionary tetraploid and/or octaploid parental species. Folia Zool. 49(2): 133-138 Abstract |
|
| Ene AC. Suciu R. 2001. Karyological investigation in natural hybrids of sturgeons of the lower Danube River. 10th European Congress of Ichthyology. Prague. (P 34) Abstract | |
|
Birstein VJ. 2002. Sturgeon Species and Hybrids: Can Hybrids Produce Caviar? Environ. Policy Law. 32: 210- 214 |
|
|
Symonová R. Flajšhans M. Gela D. Rodina M. Pelikanova Š. Rabova M. Rab P. Sturgeons are pretty polyploid: hybrid and ploidy diversity in sturgeons. International meeting on the genetics of polyploids. Lisbon, Portugal 11 - 12 november 2010 Abstract |
|
|
|
|
| 2n=60±2; 40 m+sm, 20 a, 100 FN Only macrochromosomes were counted Serebryakova EV. 1972. Some data on the chromosome complexes in Acipenseridae. In: Genetics, Selection, and Hybridization of Fish. (Ed. B.I. Cherfas). Translated from Russian by Israel Program for Scientific Translations. Keter Press Binding: Wiener Bindery Ltd. Jerusalem. pp. 98-106 |
 |
| 2n=60 Burtzev IA. Serebryakova EV. 1973. A hybrid beluga x sterlet (Huso huso (L.) x Acipenser ruthenus L., Pisces): karyology, gametogenesis and potential status. Genetics 74: s35 |
|
|
2n=117.4±1.08; 57.7±0.37 m+sm, 58.9±0.97 a, 174.3±1.41 FN |
|
| 2n=119 Ojima Y. Nakanishi Y. Takay A. 1986. Chromosomal studies of cultured cells from the hybrids between Huso huso and Acipenser ruthenus. Proc. Japan Acad. 62B(3): 87-90 Abstract |
|
| 2n=116-118 Arefjev VA. Nikolaev AI. 1993. Induced polyploidy in sturgeons: back to the problem in Russia. 31-32 pp. International Symposium on Sturgeons, Abstract Bulletin. September 6-11, 1993, Moscow-Kostroma-Moscow, Russia. Abstract |
|
|
Omoto N, Maebayashi M, Adachi S, Arai K, Yamauchi K. 2005. Sex ratios of triploids and gynogenetic diploids induced in the hybrid sturgeon, the bester (Huso huso female x Acipenser ruthenus male) Aquaculture 245: 39-47 Abstract |
|
|
|
|
| 2n=167.2±1.6; 74±0.4 m+sm, 93.6±1.4 a, 241.3±1.9 FN Arefjev VA. Nikolaev AI. 1991. Cytological analysis of the reciprocal hybrids between low- and high-chromosome acipenserids, the great sturgeon, Huso huso (L.), and the Russian sturgeon, Acipenser gueldenstaedti Brandt. Cytologia 56: 495-502 Abstract |
 |
|
|
|
| 2n=169.4±2.9; 74.2±0.7 m+sm, 95.3±2.4 a, 243.6±3.5 FN Arefjev VA. Nikolaev AI. 1991. Cytological analysis of the reciprocal hybrids between low- and high-chromosome acipenserids, the great sturgeon, Huso huso (L.), and the Russian sturgeon, Acipenser gueldenstaedti Brandt. Cytologia 56: 495-502 Abstract |
 |
|
2n=177.1±2.2; 78 m+sm, 16 a, 88 mc 260 FN |
 |
Fopp-Bayat D, Woznicki P. 2006. Verification of ploidy level in sturgeon larvae. Aquac. Res. 37: 1671-1675 Abstract |
|
|
Fopp-Bayat D, Jankun M, Woznicki P. 2007. Viability of diploid and triploid larvae of Siberian sturgeon and bester hybrids. Aquac. Res. 38: 1301–1304 Abstract |
|
Fopp-Bayat D. Jankun M. Woznicki P. Kolman R. 2007. Viability of diploid and triploid larvae of Siberian sturgeon and bester hybrids. Aquaculture Research 38: 1301-1304 Abstract |
|
m = metacentric; sm = submetacentric; a = acrocentric; mc = micro chromosomes; FN = fondamental number
Pseudoscaphirhynchus kaufmanni
| Kovalev KV. Balashov DA. Cherniak AL. Lebedeva EB. Vasil'Eva ED. Vasil'Ev VP. 2014. The karyotype of the amu darya sturgeon, Pseudoscaphirhynchus kaufmanni (Actinopterygii: Acipenseriformes: Acipenseridae). Acta Ichthyologica et Piscatoria, 44: 111-116 Abstract |